Introducing the
Compremium
Quantis ST
Clarity Through Quantification.
The Compremium Quantis ST transforms traditional palpation into a non-invasive, real-time, quantified measurement, enabling surgeons to see and measure what they feel.
It is the engineered bridge between biomechanical science and point-of-care leadership.
✓ FDA-Cleared
✓ CE-Marked
✓ CE-Marked


What the Compremium Quantis ST does
From subjective palpation to a quantified value.
Traditional palpation remains the most relied-on indicator in orthopaedic trauma, but it is subjective, inconsistent, and difficult to communicate.
In line with FDA Clearance and CE Mark, the solution:
- measures relative compartment compressibility
- provides the CP Value in real time
- is non-invasive
- enables intermittent reassessment
- captures data under a standardized and repeatable protocol
The Compremium Quantis ST enables a shift from impression to measurement, grounding decisions in facts, not feel.
Engineered to capture what surgeons feel and quantifies it.
How it works
The Compremium Quantis ST integrates:
1. Quantis ST Probe
- Captures cross-sectional ultrasound images
- Ensures consistent pressure application using defined protocols
- Designed for use with the dedicated Compremium software
2. Quantis ST Software
- Receives ultrasound and pressure signals
- Calculates the CP Value
- Displays real-time and intermittent measurement outputs
- Provides structured data for communication and clinical reporting
This integration brings precision, structure, objectivity and repeatability to soft tissue assessment, non-invasively.


Standardized Protocol
The Compremium Quantis ST provides insight via:
- automated data capture
- real-time measurement visualization
- controlled pressure application
This is Digital Palpation.
Workflow Integration
From Static to Dynamic.
Orthopaedic trauma has long centered around bone, the static element.
Yet outcomes depend equally on the dynamic behaviour of soft tissue.
The Compremium Quantis ST provides the measurement to balance this equation.
It supports:
Emergency early on-set
Optimised window of surgery
Post operative monitoring
Piece of mind at point of care
Facilitates handovers based on facts
This is the new equation of trauma success.

Where It Adds Value
Monitoring Over Time
Intermittent assessment provides documented mechanical behaviour during observation.
Optimised Window of Surgery
Quantified soft tissue insights support surgery timing.
Post-Operative Recovery
Repeatable measurement supports documentation during recovery.
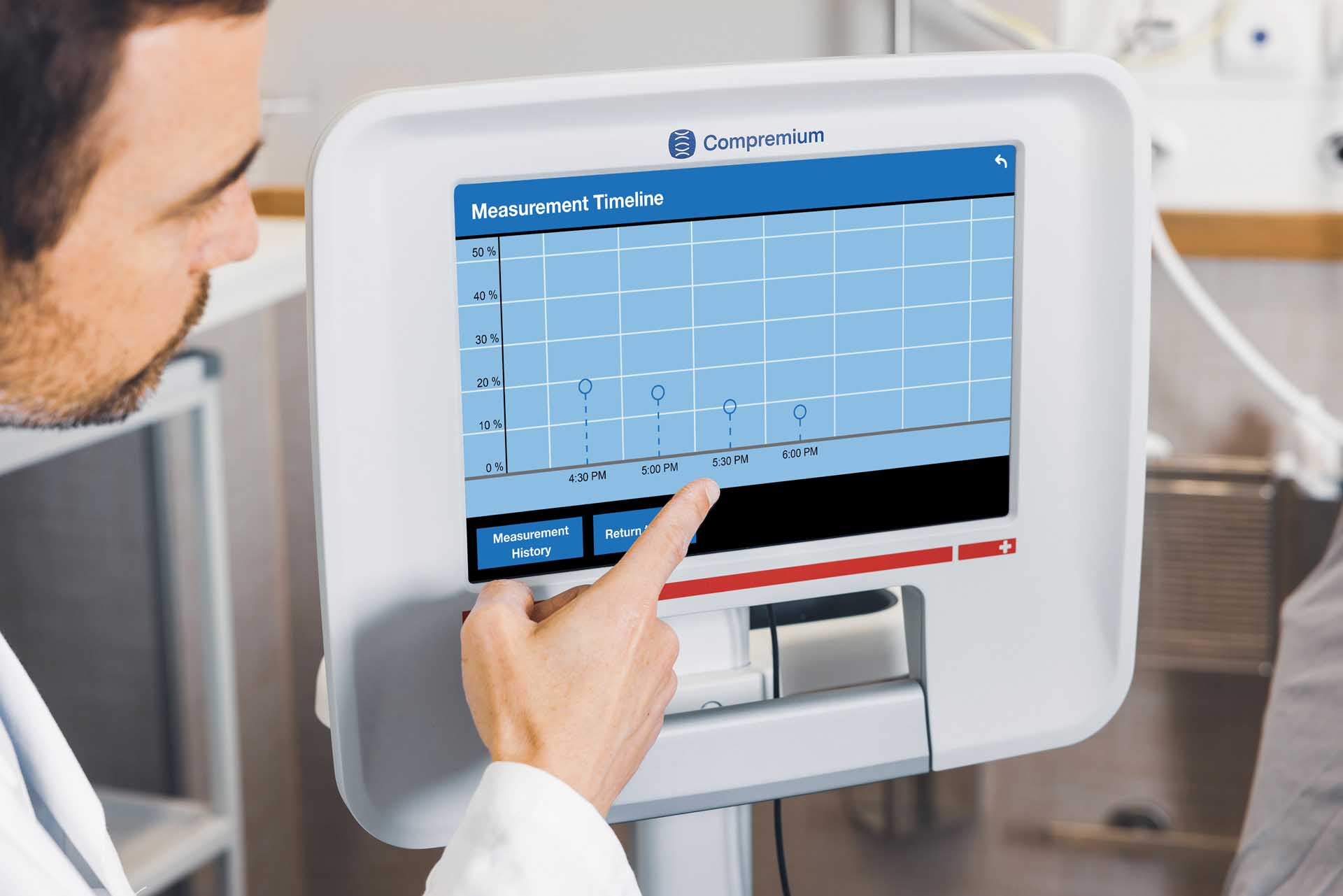

Soft tissue is now quantifiable.
Why this matters for Ortho Trauma Care
Compremium Quantis ST turns palpation into a quantified value, empowering clinicians to:
- move from “Feel” to “Facts”
- document mechanical behaviour consistently
- communicate soft tissue status clearly
Fixation alone is no longer the finish line and leaders in trauma care can now treat bone and soft tissue with equal clarity.


.jpg)


