White Paper
A New Standard for Soft Tissue Assessment: Objective, Non-Invasive, Reproducible
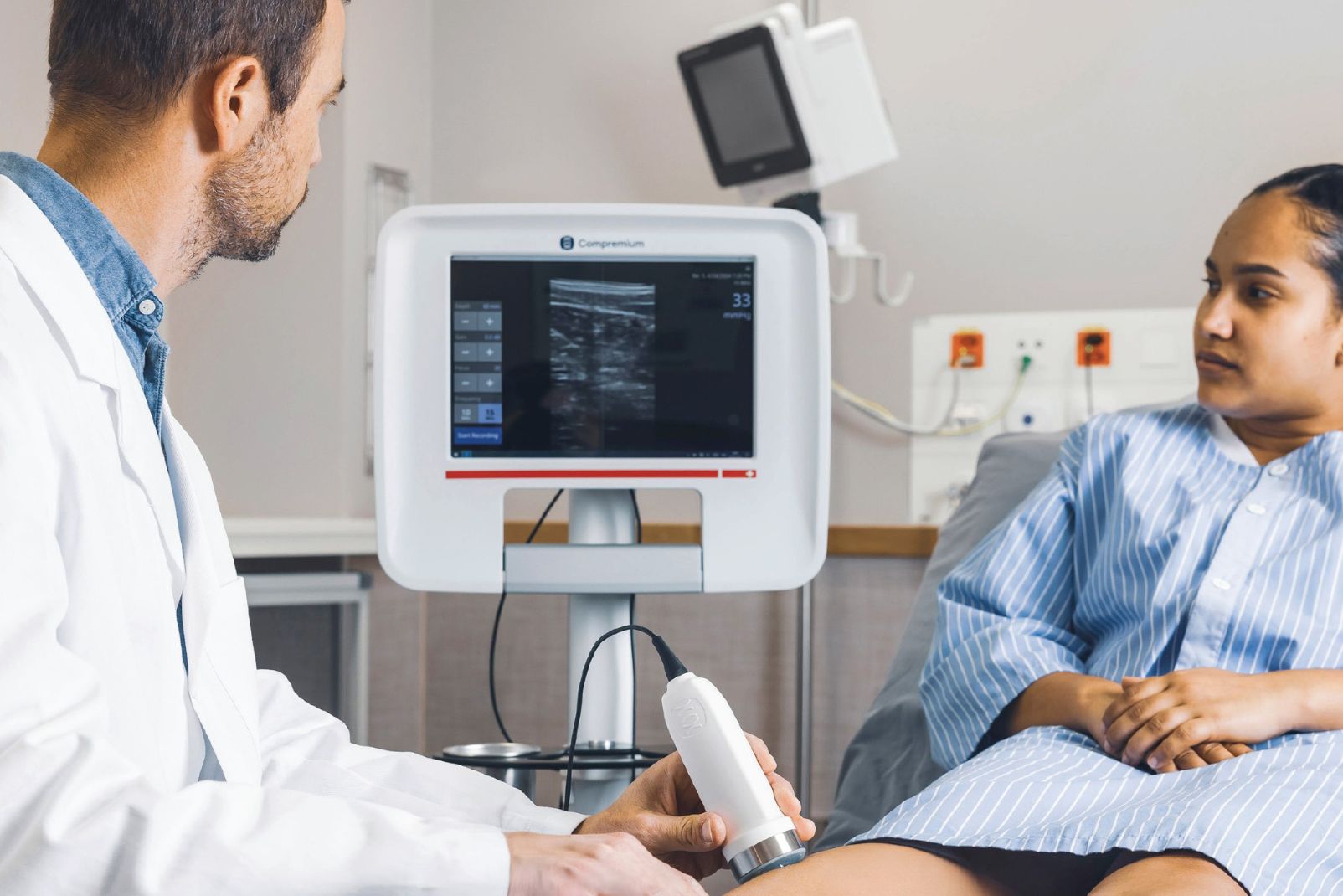
Compremium Quantis ST closes the gap in soft tissue evaluation, giving clinicians reliable data to support critical decisions.
Introduction: The Need for Better Soft Tissue Assessment in Musculoskeletal Care
Soft tissue health is crucial to recovery after musculoskeletal injury or surgery, as factors like swelling, blood flow, or delayed healing can mean the difference between a smooth recovery and long-term complications. Yet clinicians currently lack a reliable, non-invasive way to monitor it. Compremium Quantis ST fills this gap by providing objective, quantifiable, real-time data on soft tissue status non-invasively, enabling earlier intervention and reducing procedural risks. This technology enhances clinical decision-making and supports value-based care by improving outcomes, efficiency, and safety for patients.
Clinical decisions in musculoskeletal trauma often hinge on the condition of soft tissue. The current standard relies heavily on subjective palpation or invasive pressure measurements: approaches that can be limited in reliability, reproducibility, and patient comfort, with outcomes frequently affected by the level of clinical experience. In many acute settings, clinical assessments are commonly performed by less experienced healthcare providers such as residents. The gap in today’s routine care leads to uncertainty regarding surgical timing, inconsistent management strategies, and an increased risk that interventions may occur too early, resulting in unnecessary procedures like premature fasciotomies, or too late, potentially allowing complications to progress and compromise patient outcomes.
Effective soft tissue management is essential in perioperative care, where tissue condition guides surgical readiness and shapes postoperative outcomes. Injuries such as high-energy tibial plateau fractures often result in significant swelling, blistering, or compromised skin integrity, making temporizing stabilization necessary until tissues improve. The AO Foundation, recognized globally as an authority in orthopedic trauma, emphasizes that “every fracture treatment starts with soft tissue management.” This principle reflects their position that careful assessment and care of soft tissue are critical determinants of patient outcomes following musculoskeletal injury. Prioritizing soft tissue management helps reduce infection risk, supports optimal healing, and improves surgical results, setting the foundation for best practices in fracture care (1). Supporting this perspective, Borrelli concluded in his study that “significant soft tissue injuries generally accompany high-energy tibial plateau fractures. The successful management of the soft tissues surrounding the proximal tibia in the pre-, intra-, and postoperative periods is nearly as important as the accurate and thoughtful treatment of the displaced fracture.” Operating before adequate tissue recovery raises the risk of complications, infection, and poor healing, while unnecessary delays can prolong recovery and increase costs (2).
„The AO Foundation, globally recognized as an authority in orthopedic trauma, emphasizes that every fracture treatment starts with soft tissue management.“
In a recently published study of 357 patients across five Level I trauma centres, the predictive value of seven clinical signs and symptoms associated with acute compartment syndrome (ACS) was evaluated (3). The ‘seven Ps’ were pain, pallor, paraesthesia, poikilothermia, paralysis, pulselessness, and palpation. Interestingly, pain was the weakest predictor of ACS. Findings on palpation showed strong and consistent predictive value and outperformed the other six signs. Overall, the study supports objective, non-invasive monitoring of swelling and palpation findings over time.
Regulatory and clinical experts agree that accurate and timely assessment of compartment pressures is crucial for patient safety and optimal outcomes in limb compartment conditions. The FDA‘s Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee met on September 9, 2020, to evaluate the regulatory classification and safety standards for intracompartmental pressure monitor devices. The panel emphasized the value of monitoring approaches that minimize procedural risks, reflecting a general trend toward non-invasive solutions in clinical practice. The panel recognized the important role these devices play in helping clinicians detect and manage compartment pressure-related complications in a timely and effective manner, especially among patients with co-morbid conditions and those unable to reliably communicate symptoms (4).
The Compremium Quantis ST (Compremium AG, Switzerland) was developed by doctors, for doctors, to provide a non-invasive, quantitative method for assessing soft tissue compressibility across diverse clinical settings. In this white paper, acute compartment syndrome (ACS) is highlighted as a high-stakes reference condition because it is well-studied and underscores the importance of timely, accurate soft tissue evaluation. While ACS serves as a case example, Compremium Quantis ST’s clinical utility extends to a wide range of perioperative and acute care scenarios where objective assessment of soft tissue compressibility can support earlier recognition of complications, guide intervention timing, and improve patient management.
Read the full version by clicking on the button below.



