News
Compremium Receives FDA Breakthrough Device Designation for Non-Invasive Central Venous Pressure Measurement System and has also joined FDA’s TAP Program
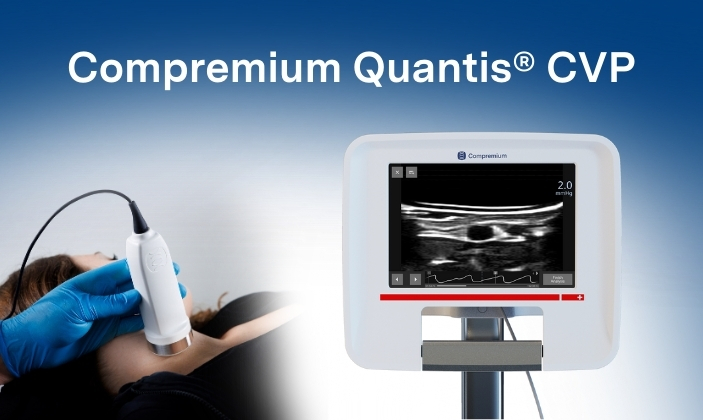
Novel solution aims to transform clinical pathways for critical hemodynamic monitoring.
MURI BEI BERN, Switzerland, Jan. 8, 2026 — Compremium AG today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its novel solution designed to directly measure central venous pressure (CVP) non-invasively. The device has also joined the FDA’s Total Product Life Cycle Advisory Program (TAP).
Volume assessment is critical for hemodynamic management of patients requiring close monitoring across acute conditions such as sepsis, shock, heart failure, venous congestion, and fluid under- or overload. Today’s gold standard relies on central venous pressure measured invasively with catheterization, which carries risks including infection and thrombosis.
Compremium Quantis CVP is a novel, non-invasive solution to quantify volume at the bedside and is designed for use in both adult and pediatric patients. Early clinical studies demonstrated promising correlation between non-invasive measurements and invasive catheter readings across clinically relevant pressure ranges. Measurements were rapid, reproducible, and achievable with limited operator training.
“Receiving Breakthrough Device Designation and joining the FDA’s Total Product Life Cycle Advisory Program reflects both the clinical importance of the unmet medical need we are addressing and the rigor of our development approach,” said Vincent Baumann, chief executive officer of Compremium. “These programs allow us to de-risk the development and shorten the time to market for Quantis CVP while strengthening our path toward clinical adoption through early engagement with FDA experts, clinicians, and payers.”
The FDA’s Breakthrough Devices Program is designed to expedite the development and review of technologies that may offer more effective diagnosis or treatment of serious conditions. The TAP Program provides coordinated FDA engagement across the product life cycle, including regulatory strategy and interactions with clinicians and payers.
Compremium Quantis CVP is built on Compremium’s established platform, components of which are already FDA-cleared and CE-marked for another clinical application.



